Prenatal Alcohol Exposure: Its Effects and Implications
September 2018/Vol.7, no.1
Ongoing research in the laboratory of Dr. Serge McGraw, from the Centre de Recherche en Reproduction et Fertilité (CRRF) of Université de Montréal, precisely identifies the impairments to young embryos caused by prenatal exposure to alcohol.
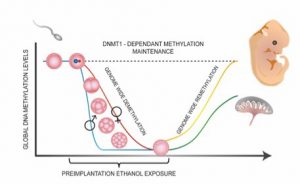
The early stages of embryonic development are critical to ensure the survival and health of the embryo. Therefore, any disturbance during this period can have serious consequences for the future newborn. In particular, poor implementation of the embryonic epigenetic program during these early days will have deleterious effects on various key processes in the development of the embryo. A crucial step in the establishment of this program is a wave of reprogramming of DNA methylation profiles that begins after fertilization of the egg. DNA methylation is, among other things, involved in transcriptional repression, genome stability, and X chromosome inactivation.
This modification is performed through specific enzymes called methyltransferases or DNMTs, and occurs at specific locations in the DNA, namely on the cytosine-guanine (CpG) sequences (Canovas and Ross 2016; McGraw et al, 2018). During the reprogramming wave, the vast majority of genome DNA methylation patterns are cleared (except for some key sequences for which maintenance of methylation is essential). Following its implantation in the uterus, the young embryo will establish its own DNA methylation imprints in order to acquire epigenetic independence required to regulate the expression of genes that are important for development of processes such as cell differentiation.
The epigenetic reprogramming period is extremely dynamic, occurring primarily during the first week of life of the embryo (Reik, Dean, and Walter 2001, Messerschmidt, Knowles, and Solter 2014).
Several studies have shown that this period is sensitive to disturbances from the external environment. Adverse maternal exposures (e.g., nutrition, medication, drugs, etc.) or specific mutations in key genes (e.g., DNMT3a) can lead to errors in the embryonic epigenetic landscape. These errors have the potential to be maintained during development and have various consequences for the health of the child (e.g., neurodevelopmental disorders) (Watkins, Lucas, and Fleming 2010).
Dr. McGraw’s ongoing research
Dr. Serge McGraw’s team is working on the adverse consequences associated with a disruption in the reprogramming wave of DNA methylation. Among other things, they want to determine the impact of prenatal alcohol exposure during the preimplantation period.
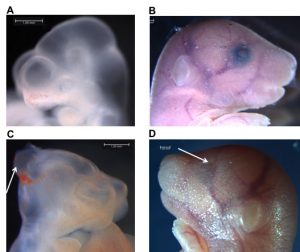
Indeed, given the large number of unplanned pregnancies (around 40%, 85 million per year worldwide) (Sedgh, Singh, and Hussain 2014), many women unwittingly expose their young embryos to high levels of alcohol during their first weeks of pregnancy. Alcohol is also known to inhibit the functioning of DNMT enzymes and to interfere with the metabolism of folate and homocysteine / methionine (Kobor and Weinberg 2011, Varela-Rey et al., 2013). This has the effect of reducing the availability of methyl groups required during epigenetic reprogramming. Dr. McGraw uses mouse models to determine permanent phenotypic, molecular and cognitive impairments initiated by prenatal exposure of young embryos to alcohol. One of the more pervasive tenets of teratology is the “all-or-none” phenomenon, which refers to the concept that embryonic exposure that occurs before organogenesis results in either no adverse embryonic outcome or in embryonic death. Dr. McGraw’s work debunks this by demonstrating that exposure to alcohol during the preimplantation period has adverse consequences on the embryo, without causing death. Moreover, his lab’s results confirm that this exposure leads to the deregulation of DNA methylation patterns in the embryonic brain at mid-pregnancy as well as an increase in morphological abnormalities (eg., delayed growth, malformations of the brain, abnormal presence of blood). The most recent experiments show that these molecular consequences have long-term impacts on brain function, as adult mice have various memory and social skill deficits, even in mice with a normal appearance.
Conclusions and perspectives
The environment to which the embryo is exposed during its first days of life may affect the long-term health of the child through the disruption of epigenetic mechanisms. By modeling the harmful effects of maternal alcohol exposure through computer-based tools, Dr. McGraw’s team hopes to understand how errors induced in the embryonic epigenetic program can lead to neurodevelopmental disorders.
References
Canovas, S., and P. J. Ross. 2016. ‘Epigenetics in preimplantation mammalian development’, Theriogenology, 86: 69-79.
Kobor, M. S., and J. Weinberg. 2011. ‘Focus on: epigenetics and fetal alcohol spectrum disorders’, Alcohol Res Health, 34: 29-37.
Legault, L. M., V. Bertrand-Lehouillier, and S. McGraw. 2018. ‘Pre-implantation alcohol exposure and developmental programming of FASD: an epigenetic perspective’, Biochem Cell Biol, 96: 117-30.
Legault, Lisa-Marie. 2018. ‘Identification de dérèglements épigénétiques embryonnaires associés à une exposition prénatale à l’alcool pendant la période préimplantatoire’. Mémoire de maîtrise.
Messerschmidt, D. M., B. B. Knowles, and D. Solter. 2014. ‘DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos’, Genes Dev, 28: 812-28.
Reik, W., W. Dean, and J. Walter. 2001. ‘Epigenetic reprogramming in mammalian development’, Science, 293: 1089-93.
Sedgh, G., S. Singh, and R. Hussain. 2014. ‘Intended and unintended pregnancies worldwide in 2012 and recent trends’, Stud Fam Plann, 45: 301-14.
Varela-Rey, M., A. Woodhoo, M. L. Martinez-Chantar, J. M. Mato, and S. C. Lu. 2013. ‘Alcohol, DNA methylation, and cancer’, Alcohol Res, 35: 25-35.
Watkins, A. J., E. S. Lucas, and T. P. Fleming. 2010. ‘Impact of the periconceptional environment on the programming of adult disease’, J Dev Orig Health Dis, 1: 87-95.
Funding sources : SickKids Foundation, CHU Ste-Justine Research Center, Université de Montréal.
Salary support : Canadian Institutes of Health Research (scholarship to LM. Legault), Fonds de recherche du Québec – Santé Junior 1 (S. McGraw).